Consistent with our mission, our broad research interests in the obstetrics-perinatology space can be collated under three main themes:
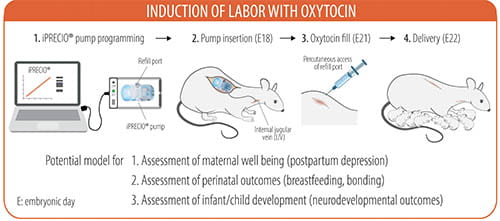
Synthetic oxytocin is used in more than 1 million laboring women in the United States annually, yet its use is not without adverse effects. Epidemiological studies suggest an association between oxytocin and neurodevelopmental disorders, but they are conflicting and do not provide mechanistic information.
To address this deficiency, we have successfully created a pregnant rat model for labor induction with oxytocin by delivering a continuously escalating dose of intravenous oxytocin through a precision delivery pump.
A major focus of our lab is to leverage this model to comprehensively investigate the direct and indirect effects of maternal oxytocin administration on the developing brain using a combination of molecular biological experiments, neuroimaging tools, and neurobehavioral assessment of the offspring.
Given the critical importance of oxytocin for satiety and energy balance, a more recent interest is to use this model to characterize the impact of perinatal oxytocin exposure on the metabolic phenotype of the offspring.
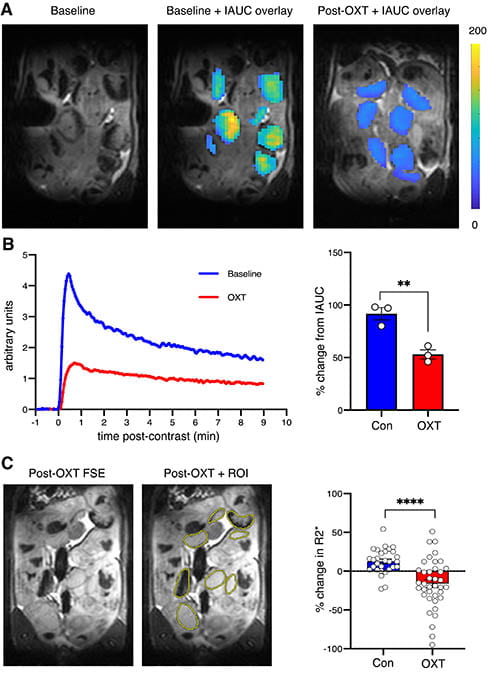
Though hypoxemic-ischemic encephalopathy after severe birth asphyxia is well-studied, not much is known about the neurodevelopmental impact of spontaneously reversible episodes of placental ischemia-hypoxemia that are more common during labor.
We recently showed, using an oxytocin-induced aberrant uterine hypercontractility paradigm in term pregnant rats, that such episodes were associated with impaired social behavior and functional brain connectivity in the male offspring.
Current work in our lab is focused on understanding the mechanisms by which transient and reversible ischemia-hypoxemia can alter neurobehavior in the offspring.
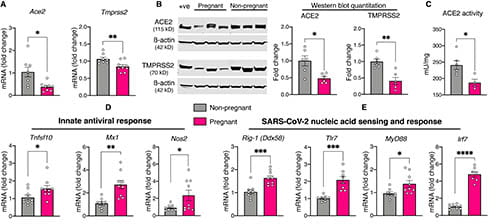
A more recent interest in our lab was fueled by the unique presentation of the COVID-19 pandemic during pregnancy compared to influenza A pandemics of the past. Our laboratory uncovered the possibility that host factors for SARS-CoV-2 cell entry may be downregulated in the nasal epithelium during pregnancy.
To follow up on these preclinical data generated from a rat model, we have embarked upon a series of foundational studies in term pregnant women to characterize the nasal epithelial transcriptome and proteome in an effort to comprehensively understand host-pathogen interaction in pregnancy.